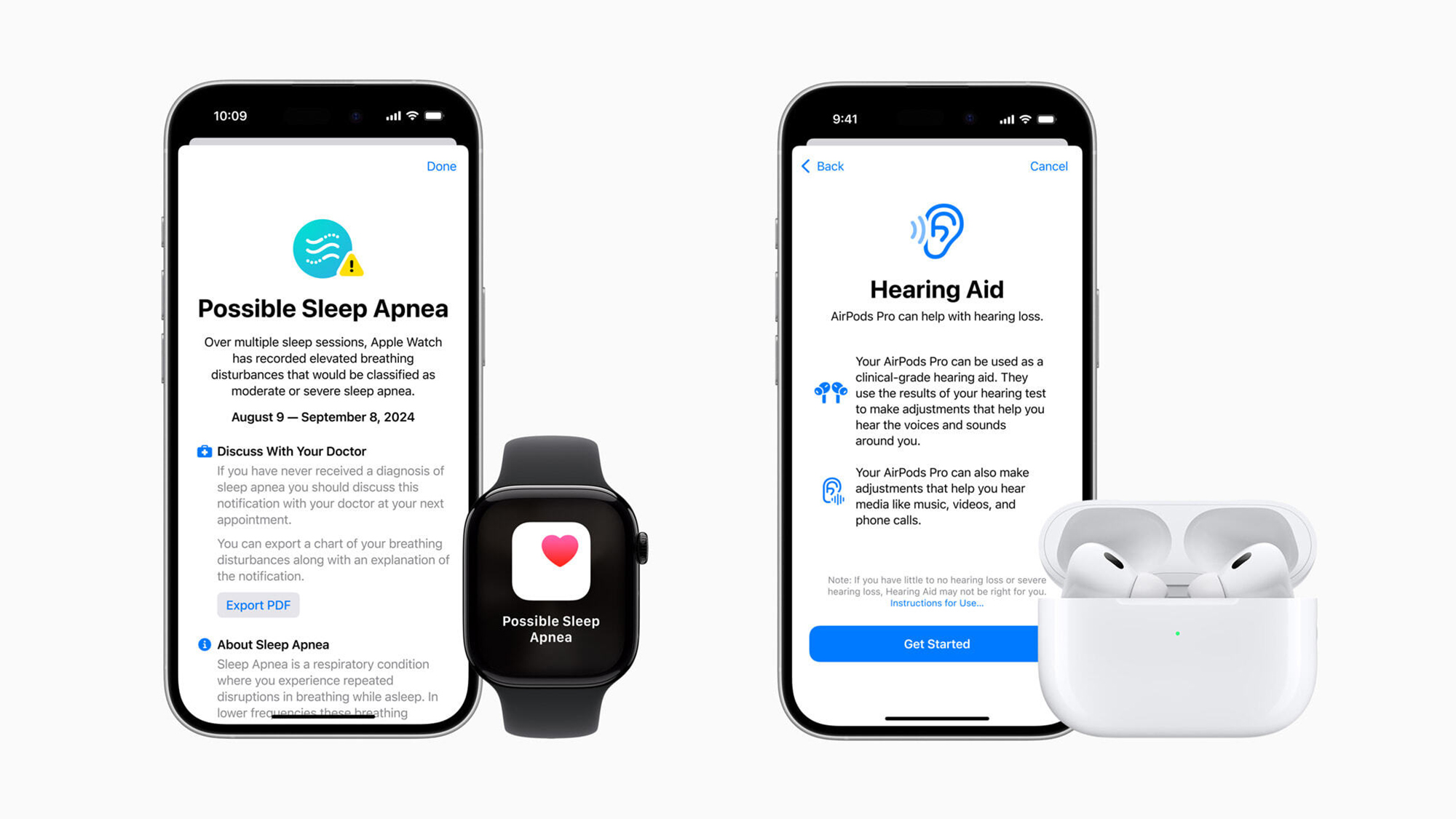

Apple has received Federal Drug Association (FDA) approval for a new Watch feature it says can detect signs of sleep apnea. The tool, available in several Watch models starting this week, uses an onboard accelerometer to measure slight wrist movements over time that may be associated with respiratory disturbances while a user is sleeping. The FDA makes clear this feature “is not intended to provide a standalone diagnosis,” or replace a doctor. Still, Apple believes the tool is important, and represents one more way its popular wrist wearable can potentially act “as an intelligent guardian for users’ health.” It’s one of several efforts by Apple to make further inroads into healthcare technology.
[ Related: Sleepy and snoring? You may have sleep apnea. ]
Millions of people with sleep apnea never receive a diagnosis
Sleep apnea is an increasingly common condition where a person temporarily stops breathing while asleep, which can cause blood oxygen levels to drop. This can lead to loud snoring or result in people waking up feeling tired. But that’s just the mild side of the spectrum. When left untreated, sleep apnea has been associated with a higher risk of type 2 diabetes and has been linked to increased risk for heart disease. Most people never even know they have the condition. The American Medical Association estimates as many as 30 million Americans may experience sleep apnea despite just six million being diagnosed. Worldwide, studies suggest sleep apnea could affect up to one billion people.
The popularity and familiarity of the Apple Watch may make it uniquely positioned to serve as a tool to help close that wide diagnosis gap. Apple’s new FDA-approved feature, called “Breathing Disturbances,” works by using an accelerator embedded in the watch to detect small movements in a user’s wrist associated with slight changes in respiratory patterns while they are asleep. The Watch compiles that data over the course of 30 days and employs an algorithm to look for patterns that suggest signs of sleep apnea. If the user is determined to be at risk, they will receive an alert on their Watch advising them to visit a physician. Users can export a PDF of their Breathing Disturbances data which they can share with their physician. Treatments for patients with diagnosed sleep apnea can include lifestyle changes in milder cases to prescriptions for a Continuous positive airway pressure (CPAP) machine in more severe cases.
“At Apple, we believe that technology can help you live a healthier life, and we’re excited to enable incredible new health capabilities for serious conditions that affect billions of people around the world, while continuing to keep user data private,” Apple Vice President of Health Sumbul Desai said in a statement.
Apple Watch cannot give medical diagnosis
It’s worth repeating that this new tool, even with the FDA approval, cannot provide an official medical diagnosis. A physical doctor would still ultimately be the one who tells patients whether or not they have the condition and how to treat it. The feature is already available on the existing Apple Watch Series 9 and Ultra 2 Models and will be available on the Series 10 watch when it releases later this week. An FDA spokesperson told Popular Science the device is intended for people above the age of 18 who have not previously received a sleep apnea diagnosis
“It is not intended to diagnose, treat, or aid in the management of sleep apnea,” the spokesperson said. “The absence of a notification is not intended to indicate the absence of sleep apnea.”
Apple’s sleep apnea tool is just the latest example of its efforts to try and turn its arsenal of wearables into functional healthcare tech. The company has already received FDA approval to use its onboard sensors to detect signs of atrial fibrillation, or A-fb. For years, many Watch users have turned to their devices to monitor their sleep, record their exercise, and even track their menstrual cycle. Some of these health tools are moving beyond the Watch.
Just this month, Apple announced they are bringing new hearing-aid-like features to its newest AirPod headset models. The new tool, which similarly received FDA approval, will use “personalized dynamic adjustments” to amplify sounds in the real world for people with mild to moderate hearing impairment. AirPods already include a clinical grade hearing test feature which users can complete in about five minutes. Studies show roughly 80% of US adults over 50 have not had their hearing checked in the past two years.
Apple is far from the only company working on integrating healthcare tech into wearable devices. What makes them unique, however, is their popularity and accessibility, particularly in the US. Apple reportedly sold around 40 million watches last year alone. Healthcare professionals have taken note. A recent report from the Wall Street Journal claims some doctors are already recommending Apple Watches informally as healthcare aides, even in cases where they haven’t necessarily received full FDA approval as medical devices. In theory, the Watch’s ubiquity and relatively low price when compared to other medical grade equipment makes them appealing for monitoring and preventative care.
But Apple’s forays into healthcare aren’t without risk. Reviewers and researchers have previously questioned the accuracy of some of its sensors, a criticism which could carry more weight when the devices are used more commonly as health aides.
New healthcare and accessibility features included wearable technology could make a meaningful difference in addressing millions of undiagnosed conditions. At the same time, healthcare professionals and regulators will need to actively take efforts to remind users of the limitations of these devices and not oversell their capabilities. Though helpful, neither Apple Watches nor AirPods can replace the role of a physician for diagnosis or treatment. In the best case scenario, the tool could give many wearers the nudge they needed to visit their doctor.