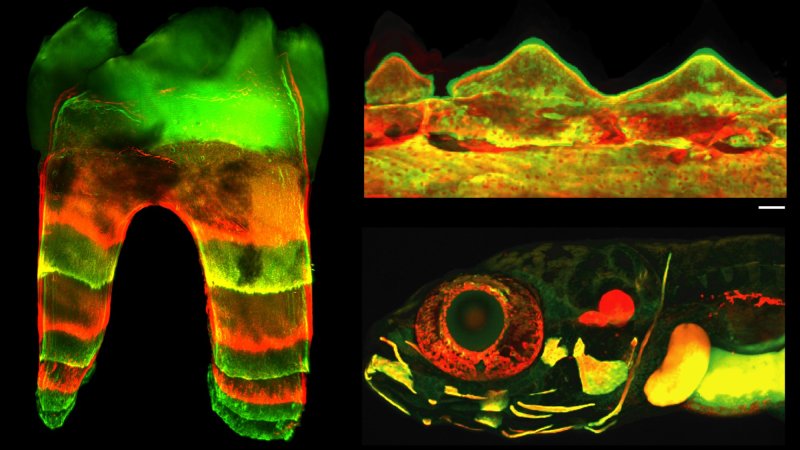
For the first time, scientists have built a detailed atlas of a zebrafish’s nerve cells. The atlas tells scientists how these special fish can fully heal a severed spinal cord and could offer clues on how to develop healing strategies for human spinal cord injuries. The findings are detailed in a study published August 15 in the journal Nature Communications.
Stem cells are not alone
Tiny and translucent zebrafish belong to an elite group of vertebrates that can fully heal a severed spinal cord. Other members of this group include tailed amphibians such as newts and axolotls and eel-like lampreys. In humans, a spinal cord injury can be completely life altering and cause permanent loss of sensation or movement. This is partially because the damaged neurons always die.
If a zebrafish’ spine is injured, the damaged neurons respond by dramatically changing their cellular functions. They take on a new and central role in orchestrating the very precise events that make it possible for them to heal. While scientists knew that zebrafish neurons survive spinal cord injury, this new research shows how.
[Related: A primitive part of the zebrafish brain helps them find their way home.]
The survival and adaptability of the severed neurons themselves is a must for the spinal cord to completely regenerate. Surprisingly, the stem cells that are capable of forming new neurons and generally thought to be central to regeneration play a more complementary role here. A different set of injury-preventing neurons leads the charge.
“We found that most, if not all, aspects of neural repair that we’re trying to achieve in people occur naturally in zebrafish,” Mayssa Mokalled, a study co-author and developmental biologist at Washington University School of Medicine in St. Louis, said in a statement. “The surprising observation we made is that there are strong neuronal protection and repair mechanisms happening right after injury.”
The team believes that these protective mechanisms allow the neurons to first survive the injury. They then adopt a “spontaneous plasticity,” or flexibility in their functions. This gives the zebrafish time to regrow the new neurons needed to make a full recovery.
“Our study has identified genetic targets that will help us promote this type of plasticity in the cells of people and other mammals,” said Mokalled.
Through mapping out the evolving roles of the various cell types involved in regeneration, the time found that the flexibility of the surviving injured neurons and their ability to immediately reprogram after injury starts the chain of events needed to completely regrow a spinal cord. If injury-surviving neurons are disabled, the fish don’t regain their normal swim capacity, even though regenerative stem cells are still there.
Toxic neurons
In humans and other mammals, if the long wiring that makes up the spinal cord is cut or crushed, it sets off a chain of toxicity events that kills the neurons, making the spinal cord environment toxic and hostile against repair mechanisms. This neuronal toxicity might provide some reasons why attempts to harness stem cells to treat spinal cord injuries in people haven’t worked out very well. Instead of focusing on regeneration with stem cells, the new paper suggests that new methods to heal spinal cord injuries in people should begin with saving the injured neurons from death.

“Neurons by themselves, without connections to other cells, do not survive,” Mokalled said. “In zebrafish, we think severed neurons can overcome the stress of injury because their flexibility helps them establish new local connections immediately after injury. Our research suggests this is a temporary mechanism that buys time, protecting neurons from death and allowing the system to preserve neuronal circuitry while building and regenerating the main spinal cord.”
There is some evidence that this healing capacity is present, but laying dormant in mammalian neurons. Finding these could be the route to new therapies, according to the team.
[Related: Swapping genes can help fruit flies regenerate cells.]
“We are hopeful that identifying the genes that orchestrate this protective process in zebrafish— versions of which also are present in the human genome—will help us find ways to protect neurons in people from the waves of cell death that we see following spinal cord injuries,” said Mokalled.
While this study is focused on neurons, spinal cord regeneration is an extremely complex process. Future work will likely delve into this new cell atlas to understand what other cell types do during spinal cord regeneration. The team is particularly interested in what part some non-neuronal cells called glia play in the central nervous system, in addition to immune cells. Ongoing studies are also underway comparing the findings in zebrafish to what is happening in mouse and human nerve tissue.