
Donor heart recipients and their doctors may soon have as much as double the standard amount of time to complete life-saving surgeries thanks to a new device dubbed HOPE. An extended time period not only could allow for more time to find feasible organ matches, but also increase the geographical range for potential transplant recipients.
Medical professionals usually have just four hours to identify, transport, and deliver a donor heart to a patient before the risk of complications begin to rise. This short window of opportunity often stems from the limitations of using static cold storage, or SCS. A long-time industry standard, a SCS involves placing a heart in a potassium solution, then housing it on ice while keeping the heart at just 4 degrees Celsius (39.2 degrees Fahrenheit). But because no active oxygenation occurs within the organ during this time, doctors only have a short window to work within before the donor heart’s health starts deteriorating. To potentially offer both doctors and patients more flexibility, researchers at Sweden’s University of Gothenburg are testing a modified version of what’s known as hypothermic oxygenated machine perfusion, or HOPE.

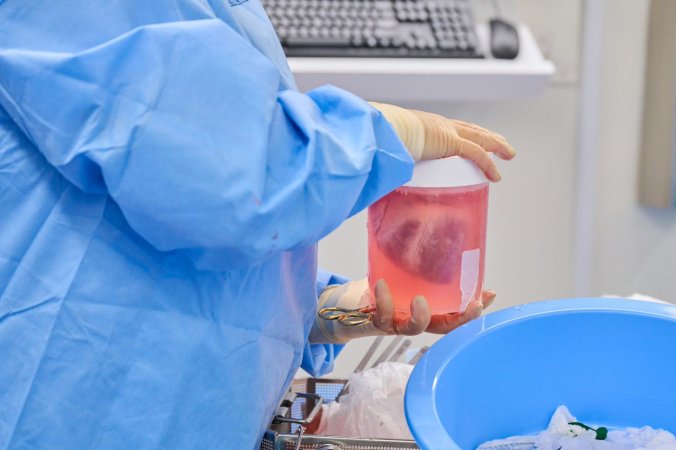
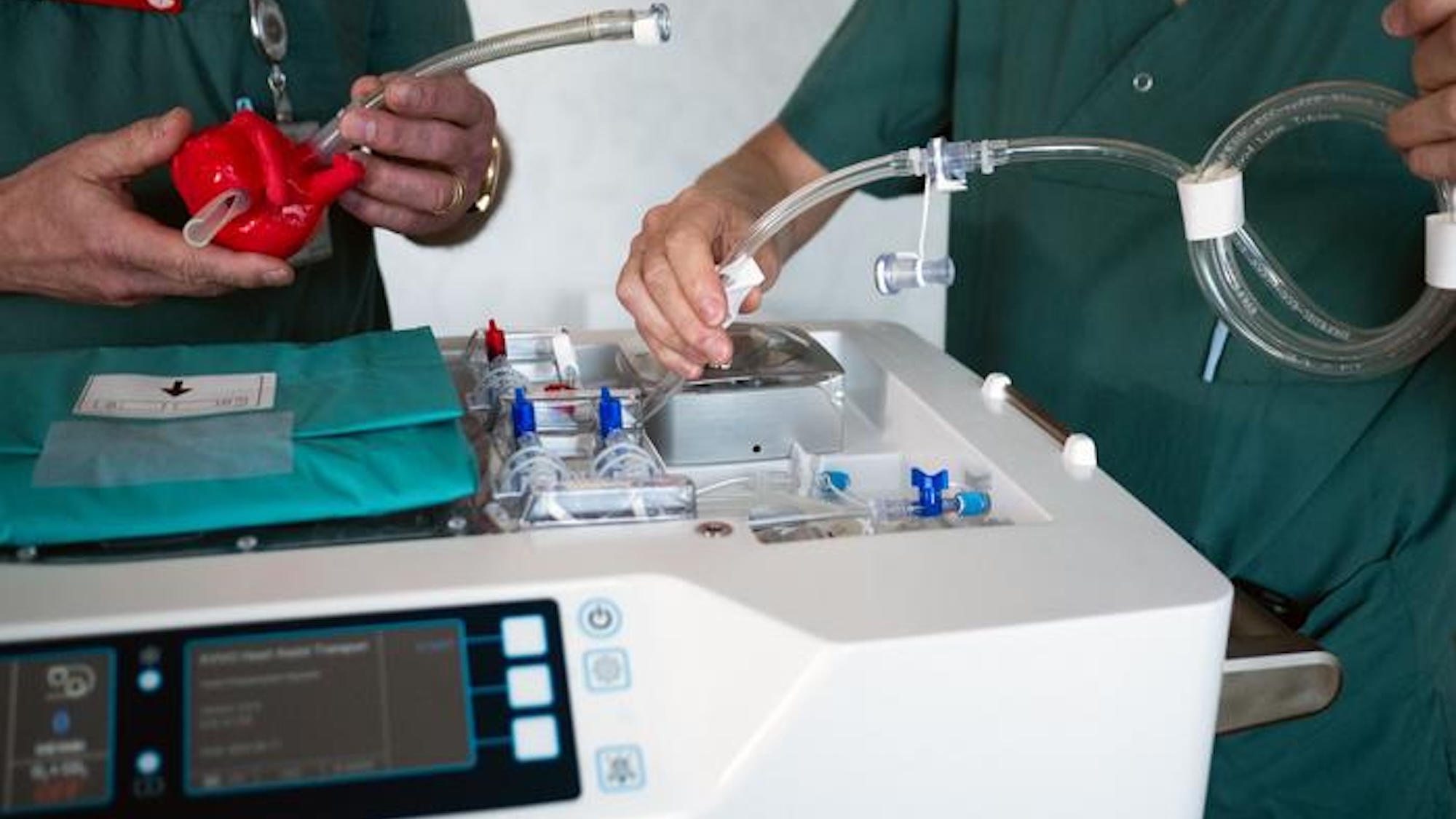
Developed by XVIVO AB, HOPE (also referred to as a “heart-in-a-box”) uses some of the same principles behind the Swedish company’s existing devices that already transport donor kidneys, lungs, and livers. Once placed in the box kept at 8 degrees Celsius (46.4 degrees Fahrenheit), a pump and tubing continuously oxygenates the organ using fluid stored in a reservoir.
“The oxygenation of the heart that takes place in the box is crucial,” said Andreas Wallinder, a cardiothoracic surgeon and XVIVO AB Medical Director, in an university announcement on August 16. “During normal cold storage and transportation, we have no oxygenation or circulation in the cells, but when we use the box, oxygenated and nutrient-rich fluid is continuously pumped through the heart, which allows the heart to function better and results in fewer complications in the recipient after the transplant.”
Wallinder’s team recently conducted a medical trial involving 204 adults registered for heart transplants across eight European countries. The results, published August 17 in The Lancet, indicate HOPE may be a major improvement compared to SCS while more than doubling the window for safely transporting organs.
[Related: First-of-its-kind implant detects and treats opioid overdoses.]
In their study, researchers randomly assigned heart transplant recipients to receive their donated organs through either the heart-in-a-box or the conventional SCS method. Within the first month of post-surgery, HOPE recipients appeared far less likely to encounter serious complications. Just 11 percent of those in the heart-in-a-box group reported severe organ failure through primary graft dysfunction, while 28 percent of SCS patients reported the issue. And although 65 percent of HOPE patients had a “serious adverse event,” that was five percent less than within the SCS group. Meanwhile, “major adverse cardiac transplant events” were just over half as likely to occur for heart-in-a-box volunteers compared to the SCS recipients.
“Used correctly, the heart-in-a-box can reduce a number of complications that otherwise often result in suffering, poor outcomes, [and] in the worst cases premature death…,” University of Gothenburg professor of transplantation surgery and study principal investigator Göran Dellgren said in the announcement.
Moving forward, researchers intend to monitor the transplant recipients over their first year post-surgery while continuing to refine the system for regulatory approval and public use.